
Decentralised (home-based) approach:
No visits to a research centre; all assessments are carried out from the participant’s home using technology
RADIAL is different from most clinical trials because it is designed to test out a way of running future trials.
The aim of RADIAL is to directly compare a decentralised (home-based) approach with both a standard, traditional (site-based) and a hybrid approach.
Approach for
the trial
To make this comparison, Trials@Home considers that an ideal focus for the RADIAL trial is people living with type 2 diabetes who are on long-acting basal insulin.
Importantly, people living with type 2 diabetes were closely consulted on the design of the trial and on the way information about the trial is explained.
RADIAL participants who enrol from this website will join the decentralised (home-based) group.

No visits to a research centre; all assessments are carried out from the participant’s home using technology

Assessments are carried out during visits to a research centre (hospital or clinic)

Some visits to the research centre are involved, but several assessments are carried out by a home nurse and via technology from the participant's home
Type 2 diabetes was chosen for the RADIAL trial because it is a very common condition which has a significant impact on people’s lives.
Much of the management of type 2 diabetes occurs at home and is also particularly suited to remote healthcare via technology. This has been widely demonstrated during the COVID-19 pandemic, when routine face-to-face medical appointments – especially for people at greatest risk from COVID-19 – had to be avoided. In addition, it is likely that technological and operational approaches used for type 2 diabetes management will also have relevance to other conditions.
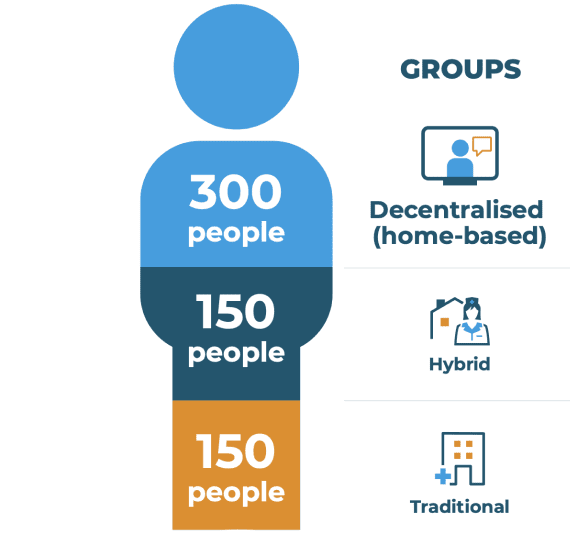
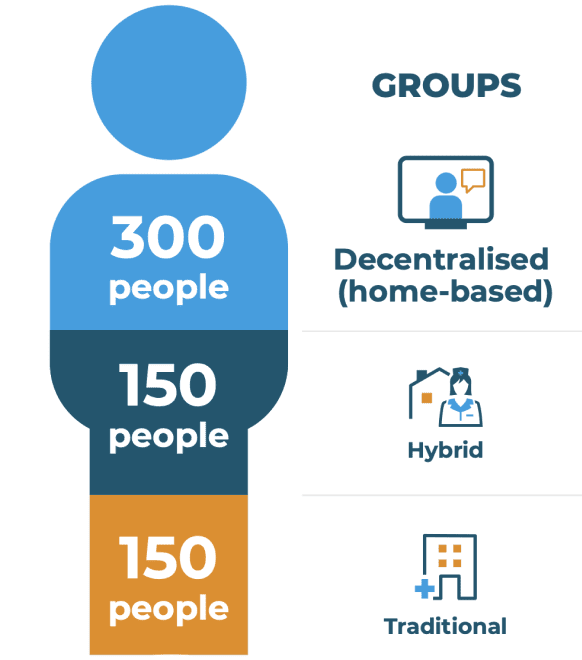
The RADIAL trial will take place in several countries across Europe. A total of 600 people will take part: 300 in the decentralised (home-based) group and 150 in each of the traditional and hybrid groups.
The screening period of the trial will last for approximately six weeks. During this period, people interested in joining will be medically assessed to see if they meet the eligibility criteria for the trial. Those who do will be invited to join the trial itself, which will last for six months.
The RADIAL trial is suitable for people aged 18+ who have lived with type 2 diabetes for at least a year and have been receiving a stable dose of long-acting basal insulin for at least 3 months.
Participants should not have achieved optimal glucose management on their current long-acting basal insulin; their HbA1c (a measure of average blood glucose over time) should be over 7 (but no higher than 10).
Participants should not be using mixed insulins and will need to avoid pregnancy. Women who are able to get pregnant should use a reliable method of contraception.
These are the main requirements for joining the trial. However, the research team will need to carry out a full medical assessment for each person who is interested in joining to confirm the trial is suitable for them.


For people living with type 2 diabetes + taking long-acting basal insulin whose blood glucose management is not currently optimal.
All participants will be switched from their current long-acting basal insulin to once-daily Toujeo® (insulin glargine 300 u/ml) and will carefully monitor their blood glucose throughout the 6-month trial.
All other medications and medical care will remain unchanged.
Data quality/completeness, trial completion rate and satisfaction of enrolled participants and trial results will be assessed to judge whether the decentralised (home-based) approach is truly a reliable and dependable way to conduct trials.


Toujeo® (insulin glargine U-300) is a once-daily, long-acting basal insulin that has been used in diabetes treatment since 2015. Toujeo® has been proven to be effective in type 2 diabetes, keeping blood glucose levels stable over 24 hours.1
Published studies have also reported a lower risk of hypos (low blood sugar levels) with Toujeo® than with some other long-acting basal insulins. 2,3,4
Previous research has shown that people living with type 2 diabetes may achieve better results when they adjust their dose of Toujeo® themselves (gradually increasing it to achieve target blood glucose levels) rather than having a healthcare professional supervise this process. This confirms that Toujeo® is safe and appropriate for people who want to manage their own insulin treatment.5