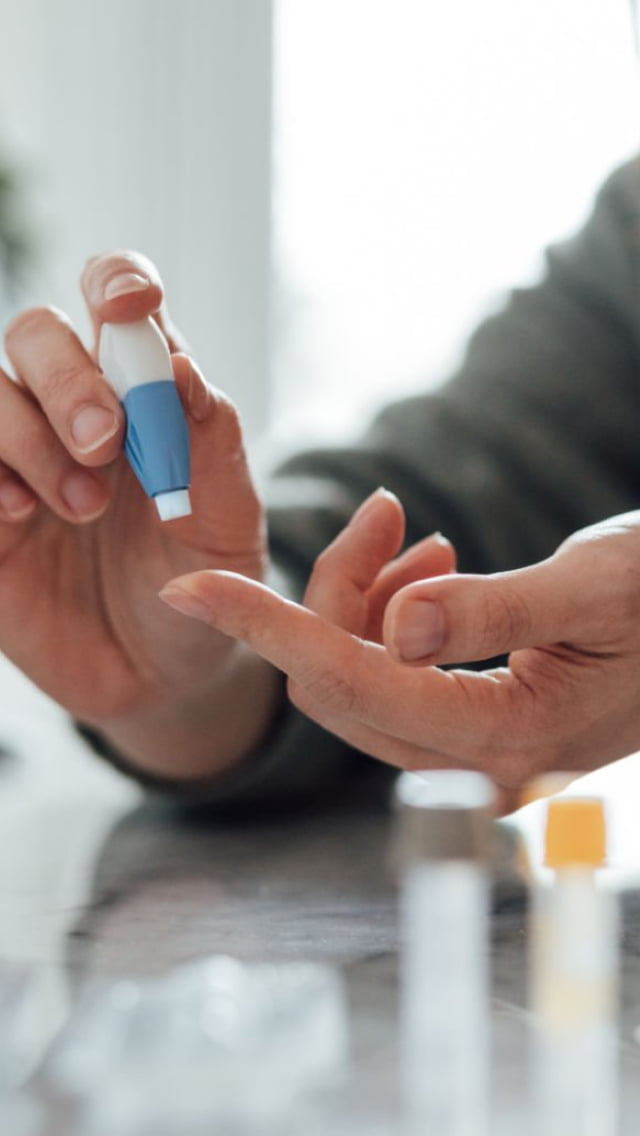
Fewer visits to the research centres
Clinical trials have always relied on the willingness of participants to make regular visits to a research centre (hospital, medical centre or clinic) for tests and assessments.
In some trials, the visits need to be frequent and can be time-consuming. This prospect can deter people from joining or completing a trial especially if they have far to travel, if the journey is difficult or expensive, if they have personal commitments like work or childcare or if they are frail, unwell, elderly or easily fatigued. The requirement for regular visits can also impact the lives of caregivers and other family members.

Perhaps it is not surprising that many clinical trials struggle to find and enrol enough participants who are willing and able to commit to travelling to a research centre on a regular basis. This can be an important reason why clinical trials are often slow to get going and also why participants may drop out before the end. The need for participants to attend a research centre has been a barrier not only to clinical research efforts but also to individuals all over the world who may really want to join a trial but can’t cope with the travel or time away from home.


Diversity among participants
Traditional clinical trials often don’t manage to achieve sufficient diversity among participants – i.e. an appropriate mix of ages, sexes, ethnicities, cultures and backgrounds reflecting the future population who will be treated with the medicine being studied.
This is a serious problem: studying a potential new medicine in a group mainly consisting of young, white men does not create the evidence needed if the medicine is ultimately also intended for women, older and frailer people, and people of other ethnic origins.
Ultimately, making clinical trials accessible to everyone who might be eligible – regardless of age, ethnicity, sex, background, health status, mobility and proximity to research centres – has become an urgent priority.
The emergence of decentralised (home-based) trials
It was not until digital technology – including the field of telemedicine – became more advanced that the idea of ‘bringing the trial to the patient’ began to emerge.
Over the past few years, researchers have begun to experiment with decentralised (home-based) clinical trials, that no longer have the hospital or clinic as the central hub of the trial, but enable patients to participate from their homes.
The massive acceleration in both progress and access to technology – combined with the inability to run trials in the normal way during the COVID-19 pandemic – set the stage for a widespread and rapid shift towards decentralised (home-based) trials.
For many trials, a fully decentralised (home-based) approach may not yet be possible as participants may require very close monitoring or need scans or procedures that depend on specialist hospital equipment. However, even in these trials, the number of visits can still be reduced by a hybrid (mixed) approach, which transfers at least some aspects of the trial to the patient’s home.


Potential benefits of decentralised (home-based) trials
Potential benefits for trial participants
- No need to travel to research centres
- Less intrusion on everyday life: ability to fit trial responsibilities around usual day-to-day activities
- Less time spent in medical environments
- Greater ability to manage own health through access to latest technology
- Ability to join clinical trials that were previously out of reach due to physical distance
Potential benefits for future patients
- Faster enrolment of clinical trials – which ultimately means faster development of effective new treatments for future patients
- Greater participant diversity in trials – which means the trial findings are likely to be relevant to a broader range of potential future patients
- Lower drop-out rates – which means the trial findings will be more trustworthy
- More efficient and timely data collection (transmitted directly from participants through secure networks in real time) with reduced risk of missing data. These benefits could also make the trial findings more trustworthy
About Trials@Home and our mission
Who we are
Trials@Home is a consortium (collaborative network) of over 30 international organisations encompassing academic institutions, patient groups, pharmaceutical companies, technology companies and associated industries that aims to bring decentralised (home-based) clinical trials into common use and ensure they are run in the best possible way. Trials@Home is supported by the Innovative Medicines Initiative (IMI), a European Union public-private partnership funding health research and innovation.
Our mission
Although many commercial organisations are now pursuing decentralised (home-based) trials of various sorts, Trials@Home is committed to defining ‘best practice’ in running decentralised (home-based) trials.
This means carefully assessing available technologies, infrastructures, operating systems and the needs and preferences of participants and researchers in order to establish an ideal ‘model’ for these types of trials which abides by all relevant laws and regulations. Ultimately these recommendations will be made available to any research team who wishes to use them.
Our goals
Specifically, Trials@Home wants to properly investigate whether decentralised (home-based) trials:
- Are reliable in the way they collect and process data and the way they monitor the progress and safety of participants
- Enable a broader range of people to take part than traditional trials can
- Are a good experience for participants
- Lead to results that are not significantly different from the results of a traditional trial when participants are given the same treatment.